
Vector illustration showing three beakers with varying solute concentrations. Clear depiction of low, medium, and high concentration levels
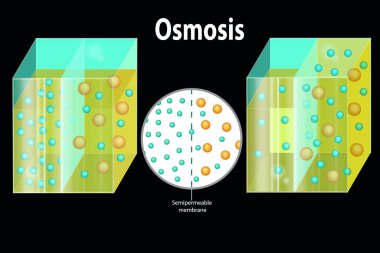
Reverse Osmosis. Process. Water passing through a semi-permeable membrane

Homogeneous, heterogeneous mixtures. sugar solution. Sand depression with water in glass

Solution science experiment. Solubility of salt and sand in water. Homogeneous, heterogeneous mixtures. Dissolving of different substances. Solute solvent chemistry explanation. Vector illustration.

Homogeneous and heterogeneous mixture. Two glasses with sugar and water, sand and water. Close-up of the molecular structure of mixtures. Vector illustration

Diffusion vs Osmosis. Solution transport process. Diffusion refers to solute move from high to low concentration. Osmosis refers to solvent move from low to high concentration. Vector illustration.

Concentration in chemistry is the abundance of a constituent in the total volume of a mixture. Two glasses with substance of Low and high concentration. Close-up of particles in liquid. Vector illustration